Mandy B. Esch, Gretchen J. Mahler, Michael L. Shuler, Body-on-a-Chip simulation with gastrointestinal and liver tissue suggests that ingested nanoparticles have the potential to cause liver injury. Lab on a Chip, 2014, 14, 3081-3092.
We present a microfluidic two-organ system with GI tract tissue and liver tissue that interact with each other through soluble metabolite exchange. The results obtained with this system suggest that two-organ systems can detect toxicity at lower concentrations of 50 nm carboxylated nanoparticles than either of the two in vitro tissues alone. In addition, the GI tract epithelium filters out nanoparticle aggregates, preventing them from entering the human body and allowing passage to single nanoparticles only.
Gretchen J. Mahler, Mandy B. Esch, Elad Tako, Shivaun D. Archer, Raymond P. Glahn, Michael L. Shuler, Oral Exposure to Nanoparticles Affects Essential Nutrient Absorption. Nature Nanotechnology, 2012, 7, 264–271 (this publication was covered by the MRS bulletin and Science Daily.)
This paper describes the effects of nanoparticle injestion on the GI tract epithelium. Using in vitro analysis and in vivo models (chicken), we found that nanoparticle injestion affects nutrient (iron) uptake negatively, and that the body compensates in the long term by increasing the surface of the its GI tract epithelium. This study looks at non-lethal, long-term effects of nanoparticles, finding that real physiologic consequences arise from nanoparticle injestion.
Mandy B. Esch, Jong Hwan Sung, Jennifer Yang, Jaijai Yu, John C. March, Michael L. Shuler, On Chip Porous Polymer Membranes for Integration of Gastrointestinal Tract Epithelium with Microfluidic ‘Body-on-a-Chip’ Devices. Biomedical Microdevices, 2012, 14 (5), 895-906
We describe a microfabrication method that allows us to shape a highly porous membrane (up to 40% porous) into a 3D macrovilli configuration where the inside of the villi is freely accessible. Since in the GI tract epithelium, stem cells move from the bottom of macrovilli to the top as they differentiate, this tissue scaffold will allow for drug uptake studies under more physiologically relevant 3D conditions. The scaffold will also allow us to investigate cancer that originates in the GI tract epithelium more closely since cancer stem cells are likely located at the bottom of macrovilli as well.
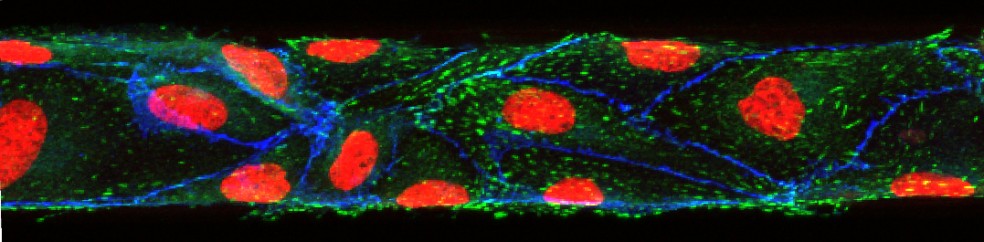
Mandy B. Esch, David J. Post, Michael L. Shuler, Tracy Stokol, Characterization of Small Diameter In Vitro Endothelial Linings of the Microvasculature. Tissue Engineering A, 2011, 17, 2965-2971
In this paper we present ultra-small microfluidic vessels (50 x 50 µm in cross section) that are fully lined with endothelial cells. We used confocal imaging to create 3D views, confirming that not only the top and bottom walls of the channels are fully lined with cells, but also the sidewalls. When visualizing the sidewalls, lines of immunostained VE-cadherin were visible, confirming that the cells had established their barrier function even on the sidewalls of the channels. Fully established barrier functions are necessary in order to use the vessels for drug uptake studies and for studies that aim to elucidate the interactions of the endothelium with circulating tumor cells
M. B. Esch, Alec Smith, Jean-Mathew Prot, Charlotta Oleaga Sancho, James Hickman, Michael L. Shuler, How Multi-Organ Microdevices Can Help Foster Drug Development. Advanced Drug Delivery Reviews, 2014, 69/70, 158-169 (invited review)
This conceptual paper outlines strategies of how to approach the design and use of multi-organ in vitro platforms for drug testing.